A Clinician’s Handbook for Childhood & Adult Immunizations in Georgia
Pathophysiology
Virus spreads when an infected person breathes out droplets and very small particles that contain the virus.
Others breathe in these droplets and particles, or these droplets and particles can land on eyes, nose, or mouth.
Droplets may contaminate surfaces touched by others.
Anyone infected with COVID-19 can spread it, even if they do NOT have symptoms.
Symptoms may appear 2-14 days after exposure to the virus.
Vaccine Description
COMIRNATY® and SPIKEVAX® are mRNA vaccines created in a laboratory to teach cells how to make a protein—or even just a piece of a protein—that triggers an immune response inside the body. The mRNA from the vaccines are broken down within a few days after vaccination and discarded from the body.
Novavax COVID-19 vaccine is a protein subunit vaccine which contains pieces (spike proteins) of the virus that causes COVID-19 along with an adjuvant that helps the immune system respond to the spike protein and develop protective antibodies to protect against COVID-19. The spike proteins by themselves cannot cause COVID-19 infection.
Indications for Use
Pfizer-BioNTech COVID-19 Vaccine (2023–2024 Formula) is authorized for children ages 6 months through 11 years; COMIRNATY is the licensed Pfizer-BioNTech product for people ages 12 years and older.
Moderna COVID-19 Vaccine (2023–2024 Formula) is authorized for children ages 6 months through 11 years; SPIKEVAX is the licensed Moderna product for people ages 12 years and older.
Novavax COVID-19 Vaccine, Adjuvanted is recommended by the CDC for use in the United States for people aged 12 years and older.
Dose and Route
- For the most up to date guidance, visit CDC’s. U.S. COVID-19 Vaccine Product Information: https://www.cdc.gov/vaccines/covid-19/info-by-product/index.html
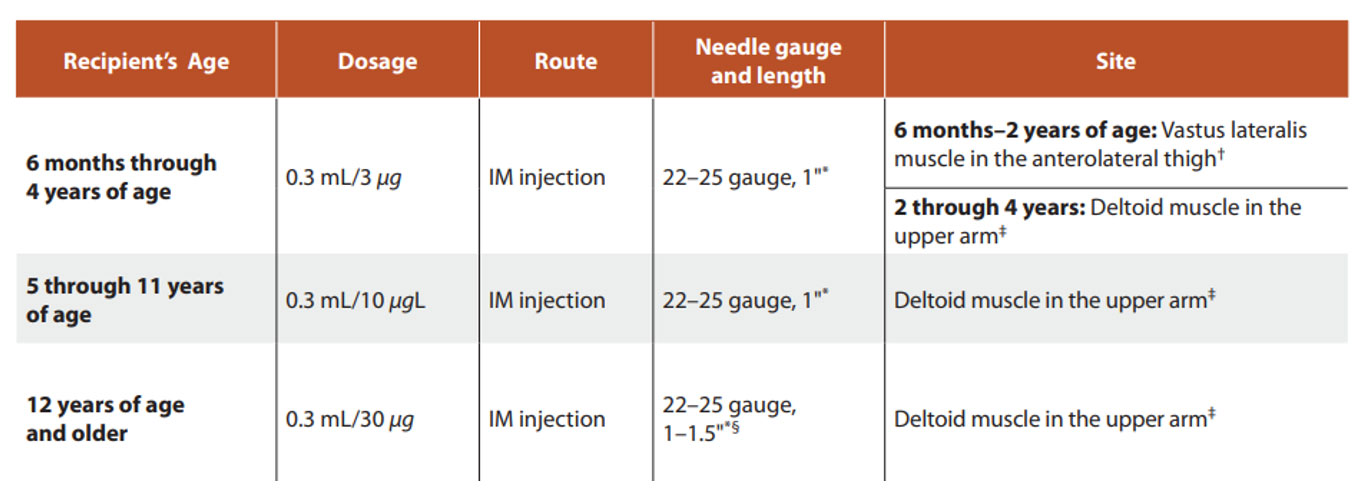
Age 6 months–4 years
- Unvaccinated:
- 2-dose series of updated (2023–2024 Formula) Moderna at 0, 4-8 weeks
- 3-dose series of updated (2023–2024 Formula) Pfizer- BioNTech at 0, 3-8, 11-16 weeks
- Previously vaccinated* with 1 dose of any Moderna: 1 dose of updated (2023–2024 Formula) Moderna 4-8 weeks after the most recent dose.
- Previously vaccinated* with 2 or more doses of any Moderna: 1 dose of updated (2023–2024 Formula) Moderna at least 8 weeks after the most recent dose.
- Previously vaccinated* with 1 dose of any Pfizer-BioNTech: 2-dose series of updated (2023–2024 Formula) Pfizer-BioNTech at 0, 8 weeks (minimum interval between previous Pfizer-BioNTech and dose 1: 3-8 weeks).
- Previously vaccinated* with 2 or more doses of any Pfizer- BioNTech: 1 dose of updated (2023–2024 Formula) Pfizer-BioNTech at least 8 weeks after the most recent dose.
Age 5–11 years
- Unvaccinated: 1 dose of updated (2023–2024 Formula) Moderna or Pfizer-BioNTech vaccine.
- Previously vaccinated* with 1 or more doses of Moderna or Pfizer-BioNTech: 1 dose of updated (2023–2024 Formula) Moderna or Pfizer-BioNTech at least 8 weeks after the most recent dose.
Age 12–18 years
- Unvaccinated:
- 1 dose of updated (2023–2024 Formula) Moderna or Pfizer- BioNTech vaccine
- 2-dose series of updated (2023–2024 Formula) Novavax at 0, 3-8 weeks
- Previously vaccinated* with any COVID-19 vaccine(s): 1 dose of any updated (2023–2024 Formula) COVID-19 vaccine at least 8 weeks after the most recent dose.
Age 19 years or older
- Unvaccinated:
- 1 dose of updated (2023–2024 Formula) Moderna or Pfizer-BioNTech vaccine
- 2-dose series of updated (2023–2024 Formula) Novavax at 0, 3-8 weeks
- Previously vaccinated* with 1 or more doses of any COVID-19 vaccine: 1 dose of any updated (2023–2024 Formula) COVID-19 vaccine administered at least 8 weeks after the most recent COVID-19 vaccine dose.
Special Considerations
Persons who are moderately or severely immunocompromised**
- Unvaccinated:
- 3-dose series of updated (2023–2024 Formula) Moderna at 0, 4, 8 weeks
- 3-dose series of updated (2023–2024 Formula) Pfizer- BioNTech at 0, 3, 7 weeks
- 2-dose series of updated (2023–2024 Formula) Novavax at 0, 3 weeks
- Previously vaccinated* with 1 dose of any Moderna: 2-dose series of updated (2023–2024 Formula) Moderna at 0, 4 weeks (minimum interval between previous Moderna dose and dose 1: 4 weeks)
- Previously vaccinated* with 2 doses of any Moderna: 1 dose of updated (2023–2024 Formula) Moderna at least 4 weeks after most recent dose.
- Previously vaccinated* with 1 dose of any Pfizer- BioNTech: 2-dose series of updated (2023–2024 Formula) Pfizer-BioNTech at 0, 4 weeks (minimum interval between previous Pfizer-BioNTech dose and dose 1: 3 weeks).
- Previously vaccinated* with 2 doses of any Pfizer- BioNTech: 1 dose of updated (2023–2024 Formula) Pfizer-BioNTech at least 4 weeks after most recent dose.
- Previously vaccinated* with 3 or more doses of any Modernaor Pfizer-BioNTech: 1 dose of any updated (2023–2024 Formula) COVID-19 vaccine at least 8 weeks after the most recent dose.
- Previously vaccinated* with 1 or more doses of Janssen or Novavax with or without dose(s) of any original monovalent or bivalent COVID-19 vaccine: 1 dose of any updated (2023–2024 Formula) of COVID-19 vaccine at least 8 weeks after the most recent dose.
- Everyone aged 65 years and older are up to date when they have received 2 updated 2023–2024 COVID-19 vaccine doses.*
People ages 65 years and older who are moderately or severely immunocompromised may receive further additional doses of any updated (2023–2024 Formula) COVID-19 vaccine (i.e., Moderna, Novavax, Pfizer-BioNTech) informed by the clinical judgement of a healthcare provider and personal preference and circumstances. Any further additional doses should be administered at least 2 months after the last updated (2023–2024 Formula) COVID-19 vaccine dose. Lean more here.
*People aged 65 years and older who have not previously received any COVID-19 vaccine doses and choose to get Novavax should get 2 doses of updated Novavax vaccine, followed by 1 additional dose of any updated 2023–2024 COVID-19 vaccine to be up to date.
Pfizer-BioNTech COVID-19 Vaccine At A Glance: Updated 2023-2024 Formula (cdc.gov)


Storage & Handling
*Click links for each Vaccine and view page 1 or see attachments
Contraindications
A severe allergic reaction (e.g., anaphylaxis) after previous dose or to a component of Pfizer-BioNTechCOVID-19 vaccine, Moderna-COVID-19 vaccine, or Novavax COVID-19 vaccine.
Precautions
History of:
- A diagnosed non-severe allergy to a component of the COVID-19 vaccine
- Non-severe, immediate (onset less than 4 hours) allergic reaction after administration of a previous dose of one COVID-19 vaccine type
- Moderate to severe acute illness, with or without fever
- Multisystem inflammatory syndrome in children (MIS-C) or adults (MIS-A)
- Myocarditis or pericarditis within 3 weeks after a dose of any COVID-19 vaccine
Special Instructions
*To monitor for allergic reactions, consider observing persons with the following precautions to a previously administered COVID-19 type after vaccination to monitor for allergic reactions:
- 30 minutes for persons with:
○ A history of a non-severe, immediate (onset within 4 hours) allergic reaction after a previous dose of one COVID-19 vaccine type
○ A history of a diagnosed non-severe allergy to a component of the COVID-19 vaccine
To monitor for syncope which might occur with any injectable vaccine, vaccination providers should consider:
- Observing for 15 minutes: All other persons (particularly adolescents)
Additional Resources
U.S. COVID-19 Vaccine Product Information | CDC
COVID-19 Vaccination Clinical and Professional Resources | CDC